Thermodynamic Equations and Functions
Explore the key thermodynamic equations and concepts that govern energy changes, spontaneity, and equilibrium in physical and chemical systems. Thermodynamic Equations and Functions
Important Thermodynamic Functions
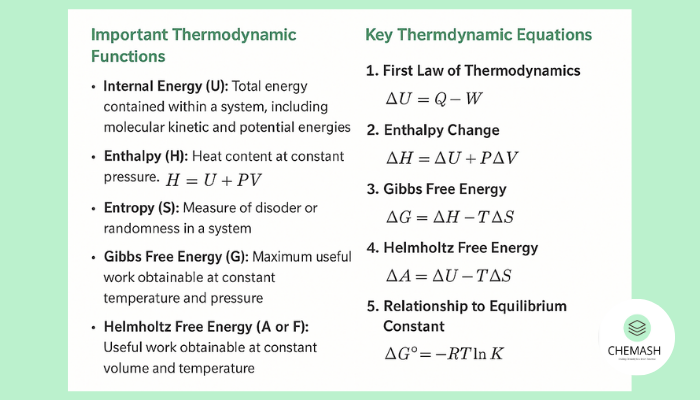
- Internal Energy (U): Total energy contained within a system, including molecular kinetic and potential energies.
- Enthalpy (H): Heat content at constant pressure, defined as H = U + PV.
- Entropy (S): Measure of disorder or randomness in a system.
- Gibbs Free Energy (G): Maximum useful work at constant temperature and pressure, G = H – TS.
- Helmholtz Free Energy (A or F): Work obtainable at constant volume and temperature, A = U – TS.
Key Thermodynamic Equations
1. First Law of Thermodynamics: ΔU = Q – W
ΔU = change in internal energy, Q = heat added, W = work done by the system.
2. Enthalpy Change: ΔH = ΔU + PΔV
3. Gibbs Free Energy: ΔG = ΔH – TΔS
4. Helmholtz Free Energy: ΔA = ΔU – TΔS
5. Relation to Equilibrium Constant: ΔG° = -RT ln K
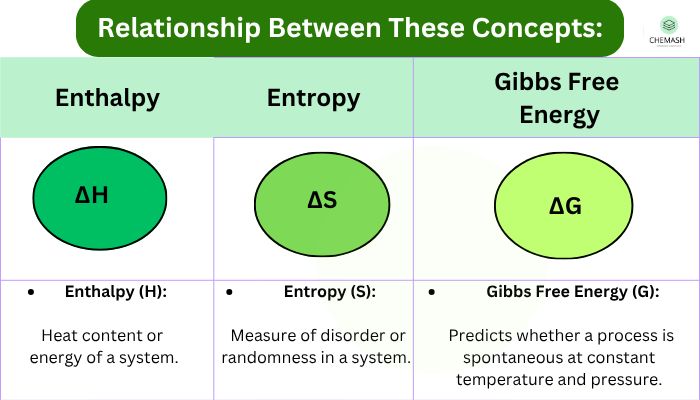
Explanation
First Law: Energy is conserved; internal energy changes depend on heat and work.
Enthalpy: Useful for reactions at constant pressure (heat absorbed or released).
Gibbs Free Energy: Determines spontaneity — a negative ΔG indicates a spontaneous process.
Helmholtz Free Energy: Useful in systems at constant volume.
Equilibrium: When ΔG° = 0, the system is at equilibrium and K determines reaction extent.
Learn more about Entropy and Spontaneity.
MCQ Quiz – Thermodynamic Functions
- Which thermodynamic function is defined as H = U + PV?
a) Internal Energy
b) Enthalpy
c) Gibbs Free Energy
d) Entropy
Answer: b) Enthalpy
Explanation: Enthalpy includes internal energy plus pressure–volume work. - What does the first law of thermodynamics state?
a) Energy can be created
b) Energy can be destroyed
c) Energy is conserved
d) Energy is infinite
Answer: c) Energy is conserved
Explanation: Energy cannot be created or destroyed, only transformed. - Gibbs free energy helps predict:
a) Temperature change
b) Volume change
c) Reaction spontaneity
d) Pressure
Answer: c) Reaction spontaneity
Explanation: A negative ΔG indicates a spontaneous reaction. - The equation ΔG° = -RT ln K relates:
a) Free energy and entropy
b) Free energy and equilibrium constant
c) Enthalpy and work
d) Internal energy and pressure
Answer: b) Free energy and equilibrium constant
Explanation: It connects Gibbs free energy with the equilibrium position of a reaction.
Frequently Asked Questions
1. What is the importance of Gibbs Free Energy?
Gibbs free energy helps predict whether a chemical reaction is spontaneous under constant temperature and pressure.
2. What does ΔH signify in thermodynamics?
ΔH (enthalpy change) represents heat absorbed or released by a system during a chemical reaction at constant pressure.
3. What happens when ΔG° = 0?
When ΔG° equals zero, the system is at equilibrium, meaning no net reaction occurs.
Up Next: Entropy – Understanding Disorder and Spontaneity
© 2025 CHEMASH | Learn more on CHEMASH Chemistry Portal

Pingback: Physical Chemistry क्या है? - CHEMASH