Thermodynamic Processes – Types, Key Equations & Real-World Applications
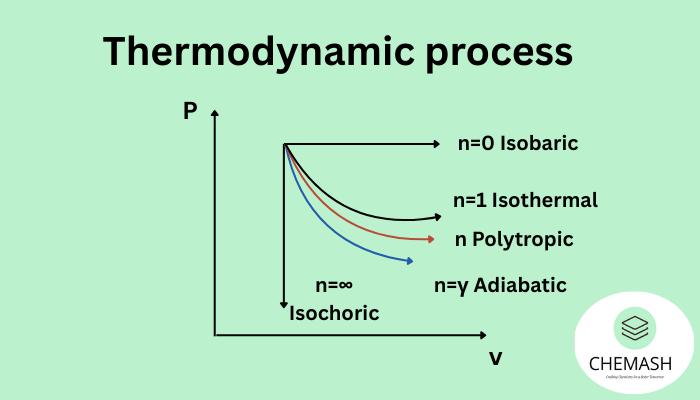
In Thermodynamic Processes, a thermodynamic process refers to the path or series of changes a system undergoes as it moves from one equilibrium state to another. These processes describe how a system’s properties such as pressure, volume, and temperature change over time due to heat exchange, work done, or internal energy changes.
Types of Thermodynamic Processes
- Isothermal Process (Constant Temperature): The temperature (T) remains constant throughout the process. Heat added to the system is used entirely to do work.
Example: Slow compression or expansion of an ideal gas in a piston at constant temperature.
Key equation: ΔT = 0; for an ideal gas, PV = constant. - Adiabatic Process (No Heat Exchange): No heat (Q) is transferred to or from the system. Changes in internal energy come solely from work done on or by the system.
Example: Rapid compression or expansion of gases where insulation prevents heat transfer.
Key equation: Q = 0; for an ideal gas, PVγ = constant (γ = Cp/Cv). - Isobaric Process (Constant Pressure): Pressure (P) remains constant while volume and temperature may change. Heat added or removed changes the system’s enthalpy.
Example: Boiling water at atmospheric pressure.
Key equation: P = constant; at constant pressure, ΔH = Q. - Isochoric Process (Constant Volume): Volume (V) remains constant, so no boundary work is done.
Example: Heating gas in a sealed, rigid container.
Key equation: V = constant, work done W = 0; thus ΔU = Q.
Other Important Thermodynamic Processes
- Polytropic Process: A generalized process where PVn = constant, with n chosen to model heat/work interactions (e.g., n=1 isothermal, n=γ adiabatic for ideal gases).
- Cyclic Process: The system returns to its initial state after a sequence of processes; total change in internal energy over one full cycle is ΔU = 0.
Significance and Applications
Understanding thermodynamic processes is crucial for analyzing engines, refrigerators, compressors, and natural phenomena. For example:
- Isothermal and adiabatic processes help in designing efficient heat engines.
- Isobaric processes are important in phase changes like boiling and melting.
- Isochoric processes are studied in sealed containers and in constant-volume calorimetry.
Note: The first law of thermodynamics (ΔU = Q − W) applies to all these processes, linking internal energy change with heat and work.
MCQ Quiz
- For an adiabatic process in an ideal gas, which statement is correct?
a) Q ≠ 0 b) PV = constant c) PVγ = constant ✅ Answer: c - In an isochoric process, the boundary work W equals:
a) PV b) 0 ✅ c) nRT Answer: b - Which process keeps pressure constant?
a) Isothermal b) Isobaric ✅ c) Adiabatic Answer: b - In an isothermal process for an ideal gas, temperature change ΔT is:
a) Positive b) Negative c) Zero ✅ Answer: c - Over a complete cycle, the change in internal energy is:
a) Positive b) Negative c) Zero ✅ Answer: c
Tip: For ideal gases, γ = Cp/Cv (≈1.4 for diatomic gases like air).
True / False
- Isothermal processes have ΔT = 0. — True ✅
- Adiabatic processes always have Q = 0. — True ✅
- In an isobaric process, ΔH equals heat at constant pressure. — True ✅
- Isochoric processes do maximum work. — False ❌ (W = 0)
- In a cycle, ΔU ≠ 0. — False ❌ (ΔU = 0 over a full cycle)
Frequently Asked Questions (FAQs)
Which thermodynamic process has no heat exchange?
An adiabatic process has no heat exchange with the surroundings, so Q = 0.
Why is work zero in an isochoric process?
In an isochoric process, the volume remains constant (ΔV = 0), hence boundary work W = PΔV = 0.
Which process is best represented by PVγ = constant?
The equation PVγ = constant represents an adiabatic process for an ideal gas.
In which process does temperature remain constant?
In an isothermal process, the temperature remains constant, so ΔT = 0.
Why is ΔU zero in a cyclic process?
In a cyclic process, the system returns to its initial state. Since internal energy is a state function, the net change is ΔU = 0.
Chemistry Branch: Physical Chemistry → Thermodynamics → Thermodynamic Processes
