Polymers are macromolecules made of repeating monomer units. They are classified by origin, molecular structure, polymerization method, intermolecular forces and biodegradability — knowledge essential for selecting materials in industry, research and product design. Types of Polymers
1. Classification Based on Origin — Natural, Synthetic & Semi-synthetic Polymers
Natural polymer examples (cellulose, proteins) and semi-synthetic derivatives
- Natural Polymers: Produced by organisms — examples: proteins, DNA, cellulose, starch, natural rubber.
- Synthetic Polymers: Chemically produced — examples: nylon, polyethylene, PTFE (Teflon), PVC.
- Semi-Synthetic Polymers: Chemically modified naturals — examples: cellulose acetate, rayon.
2. Classification Based on Structure — Linear, Branched & Cross-linked Network Polymers
How polymer architecture (linear vs branched vs cross-linked) affects properties
- Linear Polymers: Continuous chains — higher density & melting point (e.g., HDPE, PVC).
- Branched Polymers: Side chains reduce packing — lower density (e.g., LDPE).
- Cross-linked / Network Polymers: 3D covalent networks — thermosets like Bakelite and melamine.
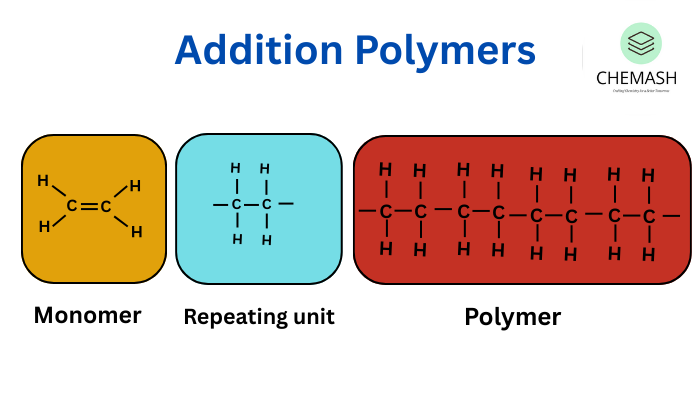
3. Classification Based on Polymerization Method — Addition (Chain) vs Condensation (Step) Polymers
Polymerization mechanisms and typical industrial examples
- Addition Polymers: Formed by chain reaction without small molecule loss — polypropylene, polystyrene, polyethylene.
- Condensation Polymers: Formed by step-growth with elimination (e.g., water) — polyesters, polyamides (Nylon-6,6), bakelite.
4. Classification Based on Intermolecular Forces & Mechanical Behavior — Elastomers, Fibres, Thermoplastics, Thermosets
Relating molecular forces to elasticity, tensile strength and thermal response
- Elastomers: Weak intermolecular forces, large elongation (natural rubber, neoprene).
- Fibres: Strong intermolecular hydrogen bonding; high tensile strength (nylon, polyester).
- Thermoplastics: Re-moldable on heating (PE, PS, PVC).
- Thermosetting Polymers: Permanently hardened by curing (Bakelite, melamine formaldehyde).
5. Classification Based on Tacticity (Stereoregularity) — Isotactic, Syndiotactic & Atactic Polymers
Effect of stereoregularity on crystallinity and physical properties
- Isotactic: Substituents on same side — high crystallinity (isotactic polypropylene).
- Syndiotactic: Alternating substituents — ordered properties (syndiotactic polystyrene).
- Atactic: Random arrangement — generally amorphous (atactic polypropylene).
6. Classification Based on Biodegradability — Biodegradable vs Persistent Polymers
Environmental impact: biodegradable polymers (PLA, PHB) vs persistent plastics (PE, PS)
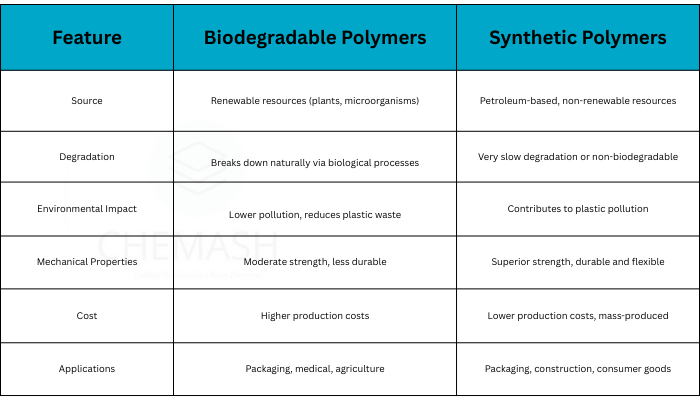
- Biodegradable Polymers: Decompose biologically — e.g., polylactic acid (PLA), polyhydroxybutyrate (PHB).
- Non-biodegradable / Persistent Polymers: Long-lived in environment — e.g., polyethylene, polystyrene.
Quiz: Types of Polymers (MCQs)
- Q1. Which of the following is a thermoplastic polymer?
A) Bakelite B) Melamine C) Polyvinyl chloride (PVC) D) Urea-formaldehyde
Answer: C — PVC is a thermoplastic that softens on heating and can be reshaped. - Q2. Which of the following is a natural polymer?
A) Nylon B) Polyethylene C) Cellulose D) Polystyrene
Answer: C — Cellulose is a naturally occurring polysaccharide in plants. - Q3. Isotactic polymers have:
A) Random arrangement of groups B) Groups on opposite sides C) All groups on same side D) No substituents
Answer: C — Isotactic polymers have substituents arranged on the same side of the chain. - Q4. Which is NOT a biodegradable polymer?
A) Polyethylene B) PLA C) PHBV D) Polyglycolide
Answer: A — Polyethylene is non-biodegradable and contributes to persistent plastic pollution.
Frequently Asked Questions (FAQ)
Q: What is the main difference between addition and condensation polymers?
A: Addition (chain) polymers form by successive addition of monomers with no small-molecule by-product; condensation (step-growth) polymers form with elimination of small molecules such as water.
Q: Are biodegradable plastics always environmentally safe?
A: Not necessarily. Biodegradation depends on conditions (temperature, microbes, composting). Proper disposal and industrial composting facilities often required for full breakdown.
Q: Can we convert thermosets into thermoplastics?
A: Thermosets undergo irreversible cross-linking; they cannot be remelted like thermoplastics. Recycling often requires mechanical grinding or chemical routes, not simple remelting.
Published by CHEMASH • Last updated: 11 Sep 2025
