UV–Visible Spectroscopy
UV Visible Spectroscopy is one of the most important analytical techniques in physical and organic chemistry. It is extensively used in Boards, JEE, NEET and also in industrial and research laboratories.
Table of Contents
- Introduction
- Principle
- Beer–Lambert Law
- Electronic Transitions
- Chromophores & Auxochromes
- Spectral Shifts
- Instrumentation
- Applications
- Limitations
- Numerical Practice
- MCQs
- Exam Tips
1. Introduction
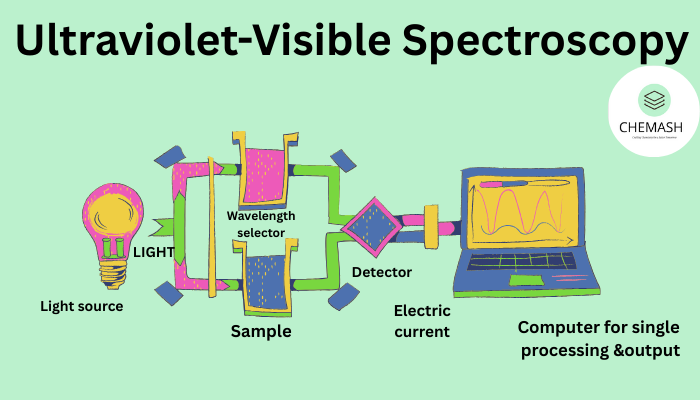
UV–Visible spectroscopy involves the absorption of electromagnetic radiation in the 200–800 nm region. This absorption results in the excitation of electrons from lower energy orbitals to higher energy orbitals.
Key Idea: Absorption of UV–Visible radiation corresponds to electronic transitions.
2. Principle of UV–Visible Spectroscopy
When radiation passes through a sample, specific wavelengths are absorbed depending upon the molecular structure. The remaining light is transmitted and measured.
The absorbance depends on:
- Nature of compound
- Concentration of solution
- Path length of cuvette
3. Beer–Lambert Law
The quantitative aspect of UV–Visible spectroscopy is governed by the Beer–Lambert Law:
A = ε × c × l
| Symbol | Meaning |
|---|---|
| A | Absorbance |
| ε | Molar absorptivity |
| c | Concentration |
| l | Path length |
4. Electronic Transitions
| Transition | Energy | Example |
|---|---|---|
| σ → σ* | Very High | Alkanes |
| n → σ* | High | Alcohols |
| π → π* | Moderate | Alkenes |
| n → π* | Low | Carbonyls |
5. Chromophores & Auxochromes
Chromophore: Part of molecule responsible for absorption.
Auxochrome: Group that enhances intensity or shifts λmax.
6. Spectral Shifts
- Bathochromic shift: Red shift
- Hypsochromic shift: Blue shift
- Hyperchromic effect: Increase in absorbance
- Hypochromic effect: Decrease in absorbance
7. Instrumentation
- Light source (Deuterium & Tungsten)
- Monochromator
- Quartz cuvette
- Detector
- Recorder
8. Applications
- Drug analysis
- Reaction kinetics
- Biochemical analysis
- Industrial quality control
9. Limitations
- Only chromophoric compounds
- Low selectivity
- Solvent interference
10. Numerical Practice (Boards / JEE / NEET)
Numerical 1:
Absorbance of solution is 0.8, ε = 1600 L mol⁻¹ cm⁻¹, l = 1 cm. Calculate concentration.
Solution:
c = A / (εl) = 0.8 / (1600 × 1) = 5 × 10⁻⁴ mol L⁻¹
Numerical 2 (JEE):
If concentration doubles, absorbance becomes?
Absorbance also doubles (directly proportional).
11. MCQs with Answers
Q. Which transition absorbs at longest wavelength?
A) σ → σ*
B) n → σ*
C) π → π*
D) n → π*
Correct Answer: D
Explanation: Lowest energy transition.
12. Exam Tips & Common Mistakes
- Always check units in numericals
- Beer–Lambert law valid only for dilute solutions
- Remember transition energy order
© CHEMASH – Chemistry Made Easy | Physical Chemistry | External Reference

Pingback: Infrared Spectroscopy – Principle, Instrumentation, Interpretation .