Werner’s Theory of Coordination Compounds
Table of Contents
Alfred Werner, a Swiss chemist, proposed the first successful theory to explain the structure of coordination compounds in 1893. Werner’s theory revolutionized inorganic chemistry and earned him the Nobel Prize in Chemistry in 1913.
Key Points of Werner’s Theory
- Primary and Secondary Valencies:
- Primary valency = oxidation state of the central metal ion, satisfied by anions.
- Secondary valency = coordination number, satisfied by ligands.
- Coordination Number: Number of ligands attached directly to the metal ion.
- Spatial Arrangement: Ligands are arranged in definite geometries (octahedral, tetrahedral, square planar).
- Isomerism: Explained existence of structural and stereoisomers.
- Complex Ion Formation: Central metal ion + ligands → stable coordination complex.
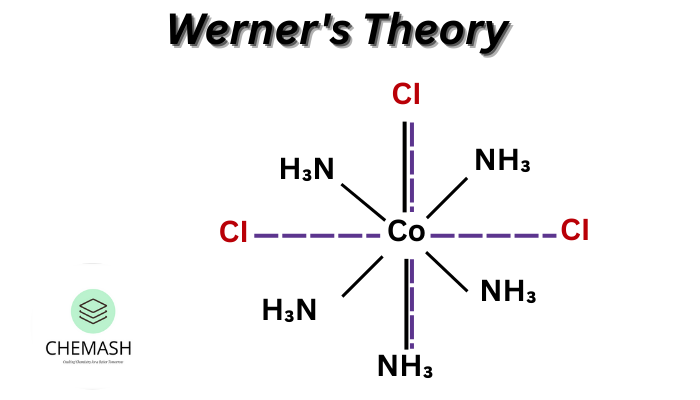
Illustration
Example: [Co(NH3)6]Cl3
- Primary valency = +3, satisfied by 3 Cl⁻ outside the coordination sphere.
- Secondary valency = 6, satisfied by 6 NH₃ ligands inside the coordination sphere.
Significance of Werner’s Theory
- Explains difference between simple salts and coordination compounds.
- Introduced coordination number and molecular geometry.
- Explains isomerism in complexes.
- Foundation for modern coordination chemistry.
Quiz: Test Your Understanding
- What are primary and secondary valencies according to Werner theory?
- How does Werner’s theory explain isomerism?
- What is the coordination number in [Co(NH3)6]Cl3?
- Who proposed the theory of coordination compounds?
- What is the difference between ligands and anions?
Answers
- Primary valency = oxidation state satisfied by anions; secondary valency = coordination number satisfied by ligands.
- By different arrangements of ligands around metal ion → explains isomers.
- 6
- Alfred Werner
- Anions satisfy primary valency (outside), ligands satisfy secondary (inside coordination sphere).
(MCQs)
- According to Werner’s theory, primary valencies are:
a) Ligands
b) Coordination number
c) Oxidation state of metal
d) Neutral molecules - Secondary valencies correspond to:
a) Oxidation state
b) Coordination number
c) Number of anions
d) Valence electrons - Father of coordination chemistry is:
a) Berzelius
b) Lewis
c) Werner
d) Pauling - Coordination number of Co in [Co(NH3)6]Cl3:
a) 3
b) 6
c) 9
d) 1 - Ligands are:
a) Anions outside
b) Molecules/ions attached to metal
c) Always negative
d) Unrelated
FAQs on Werner’s Theory
Q1: What is the difference between primary and secondary valency?
A: Primary = oxidation state, satisfied by anions. Secondary = coordination number, satisfied by ligands.
Q2: How does Werner theory explain isomerism?
A: Different spatial arrangements of ligands explain structural and stereoisomers.
Q3: What is the significance of Werner theory?
A: It established coordination chemistry, explained geometry, valency, and isomerism of complexes.
Related reading: Isomerism in Coordination Compounds | Alfred Werner (Wikipedia)
