The Equilibrium Constant (K) in chemistry is a key concept that defines the ratio of product concentrations to reactant concentrations at equilibrium for a reversible reaction. The equilibrium constant provides a quantitative measure of how far a reaction proceeds and helps chemists predict the reaction’s direction under specific conditions.
Equilibrium Constant (K)
ContentsIntroductionDefinition & ExpressionTypes (Kc, Kp)Relation: Kp & KcSignificance of KReaction Quotient (Q)Worked ExampleMCQsQuizFAQsReferences
Introduction
The Equilibrium Constant (K) is a dimensionless (or unit-specific) value that quantifies the ratio of products to reactants at equilibrium for a reversible chemical reaction. It tells you how far the reaction proceeds under given conditions and helps predict the direction the reaction will move when disturbed.
Definition and Expression
For a general reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant K (in concentration form, Kc) is defined as:
K = ([C]^c × [D]^d) / ([A]^a × [B]^b)
Here [X] denotes the molar concentration of species X at equilibrium and a,b,c,d are stoichiometric coefficients.
Types of Equilibrium Constants
- Kc: Equilibrium constant in terms of molar concentrations (M).
- Kp: Equilibrium constant using partial pressures for gaseous species (atm or bar).
- Kx / Other: Mole-fraction-based forms or activity-based constants are used in specialized contexts.
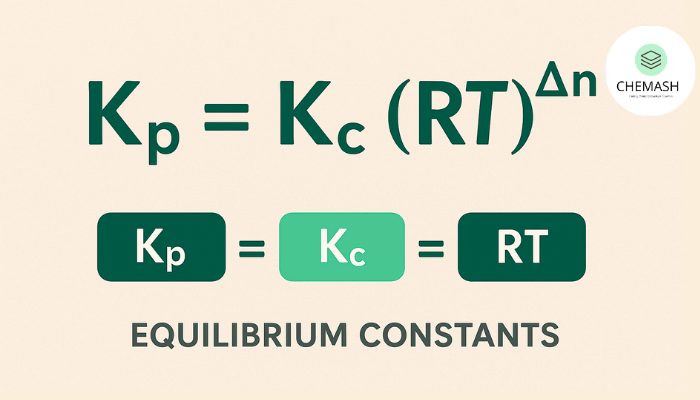
Relationship Between Kc and Kp
For gaseous reactions:
Kp = Kc (RT)^{Δn}
Where:
R= universal gas constant (0.082057 L·atm·mol−1·K−1),T= temperature in Kelvin,Δn= (moles gaseous products) − (moles gaseous reactants).
Significance of the Equilibrium Constant
- Magnitude of K indicates position of equilibrium:
K ≫ 1— products favored (reaction proceeds near completion).K ≪ 1— reactants favored (little product formed).K ≈ 1— comparable amounts of reactants and products at equilibrium.
- Predicts direction: Comparing reaction quotient
QwithKshows whether the system will move forward or backward. - Depends on temperature: K changes with temperature; heating/cooling shifts K depending on reaction enthalpy.
Reaction Quotient (Q)
Q uses the same mathematical form as K but with initial (or any non-equilibrium) concentrations/pressures. Compare Q with K:
Q < K→ reaction proceeds forward (makes more products).Q > K→ reaction proceeds backward (makes more reactants).Q = K→ system is at equilibrium.
Example Calculation
Consider the Haber-type reaction (gases):
N2(g) + 3H2(g) ⇌ 2NH3(g)
If at equilibrium:
[N2] = 0.50 M[H2] = 1.50 M[NH3] = 0.75 M
Then
Kc = [NH3]^2 / ([N2] × [H2]^3) = (0.75)^2 / (0.50 × (1.50)^3) = 0.5625 / 1.6875 = 0.333
MCQs: Quick check
- Which expression correctly gives
KforaA + bB ⇌ cC + dD?- a) ([A]^a [B]^b)/([C]^c [D]^d)
- b) ([C]^c [D]^d)/([A]^a [B]^b) ✅
- The relationship between Kp and Kc contains which factor?
- a) (RT)^Δn ✅
- b) (PV)^n
- If Q < K, the reaction will:
- a) move backward
- b) move forward ✅
Short Quiz
- What is the practical meaning of a very large K value?
- How do you get Kp from Kc for gas reactions?
- Given initial concentrations, how would you use Q to tell the reaction direction?
Answers (brief):
- Products are strongly favored (reaction proceeds nearly to completion).
- Use
Kp = Kc (RT)^{Δn}with Δn = (products gas moles − reactants gas moles). - Compute Q using initial values: if Q < K → forward, if Q > K → backward, if Q = K → equilibrium.
Frequently Asked Questions
Q: Does K have units?
A: Formally K can be unitless when activities are used; when concentrations are used you may see units, but many textbooks present K as dimensionless by dividing by standard states.
Q: Why does K change with temperature?
A: K depends on the reaction’s enthalpy. Changing temperature shifts the equilibrium and thus the ratio of products to reactants at equilibrium (Le Chatelier & van ‘t Hoff relation).
Q: Can catalysts change K?
A: No — catalysts speed up the attainment of equilibrium by increasing both forward and reverse rates, but they do not change the equilibrium constant K.
Summary
The equilibrium constant K is a compact, quantitative description of where a reversible chemical reaction lies at equilibrium. It helps chemists predict reaction behavior, choose conditions to favor products or reactants, and interpret how temperature or pressure changes will alter a system.
