General Characteristics of Solids
Solids represent one of the fundamental states of matter characterized by closely packed particles, definite shape, and strong intermolecular forces. In addition, these features set solids apart from liquids and gases and play a crucial role in material science, construction, electronics, and everyday applications.
1. Definite Shape and Volume
Solids have a rigid structure with fixed shape and volume. Unlike liquids and gases, they do not adapt to the container. This behavior is due to:
- Strong intermolecular forces
- Minimal compressibility
- Particles at fixed positions
2. Rigidity and Incompressibility
Solids are rigid and resist compression because particles are tightly packed, leaving little space for movement under pressure.
3. High Density
Compared to liquids and gases, solids typically have higher densities due to their compact structure. For instance:
- Metals like iron and copper have high density due to metallic bonding
- Osmium is often recognized as the densest naturally occurring solid
4. Strong Intermolecular Forces
Atoms or molecules in solids are held by ionic, covalent, metallic, or van der Waals forces—contributing to stability, mechanical strength, and durability.
5. Fixed Lattice Arrangement
Crystalline solids have atoms arranged in a repeating 3D pattern (lattice), which determines their physical and chemical behaviors.
6. Melting Point
Most solids have a sharp melting point because strong bonds must be broken. However, amorphous solids like glass melt gradually over a temperature range.
7. Low Kinetic Energy
Solid particles only vibrate around fixed positions with minimal kinetic energy. As temperature increases, these vibrations intensify and eventually lead to melting.
8. Anisotropy (in Crystalline Solids)
Crystalline solids often exhibit anisotropy, meaning their properties change based on direction. This is due to directional dependence of atom/lattice arrangement.
9. Types of Solids
Solids are broadly classified into:
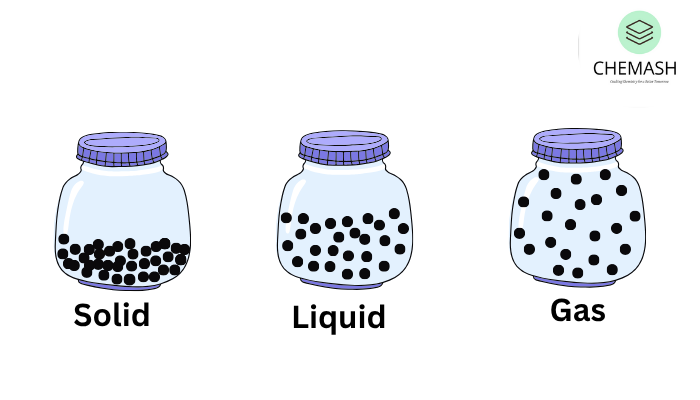
- Crystalline Solids: Ordered and periodic atomic arrangement (e.g. NaCl, diamond)
- Amorphous Solids: Irregular structure, no sharp melting point (e.g. glass, plastic)
Conclusion: Solids are characterized by structural rigidity, strong intermolecular interactions, and distinctive physical properties. Therefore, understanding these traits is critical in material development, electronics, and structural engineering.
