Group 2: Alkaline Earth Metals
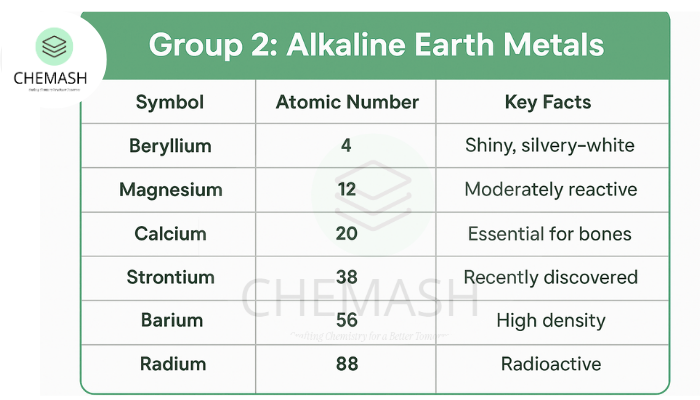
Group 2 elements of the periodic table are known as the Alkaline Earth Metals. This group includes Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra). These metals are shiny, silvery-white, and moderately reactive — less than Group 1 alkali metals.
Electronic Configuration & General Features
All alkaline earth metals have two electrons in their outermost shell, with general configuration ns^2. Key features:
- Readily lose two valence electrons to form
M2+ions. - Exhibit a stable oxidation state of +2.
- Higher ionization energy and smaller radii than Group 1 Alkali Metals.
Physical Properties
- Harder with higher melting points than alkali metals.
- Show metallic luster; moderately reactive.
- Density increases down the group (Be is an exception).
- Good conductors of heat and electricity.
Chemical Properties
- With Water: React to form hydroxides and H2 gas (Be does not react easily).
- With Oxygen: Form basic oxides (
MO). - With Halogens: Form ionic halides (
MX2), except Be halides (covalent). - Thermal Stability: Carbonates & nitrates become more stable down the group.
Important Compounds & Uses
- CaCO3: In limestone, cement, chalk, dietary supplement.
- MgSO4 (Epsom salt): Medicine & agriculture.
- BaSO4: Radiocontrast agent in imaging.
- Be alloys: For springs, tools, electrical contacts.
- Ra: Historically used in luminous paints (now discontinued).
Trends in Group 2
– Atomic/ionic radii ↑ down the group
– Ionization energy ↓ → Reactivity ↑
– Metallic character ↑
– Carbonates & nitrates become more thermally stable
– Hydroxide solubility ↑ down the group
MCQ Quiz: Test Your Knowledge
- General electronic configuration of Group 2 elements is:
a) ns1 b) ns2 c) np2 d) (n-1)d10ns2
Answer: b) ns2 - Which alkaline earth metal does not react with water easily?
Answer: Beryllium (Be) - Formula of calcium oxide is:
Answer: CaO - Which property increases down Group 2?
Answer: Reactivity with water
FAQs on Alkaline Earth Metals
Q1: Why are Group 2 elements called alkaline earth metals?
Ans: Because their oxides and hydroxides are alkaline in nature and occur in earth’s crust.
Q2: Which is the most abundant alkaline earth metal in the human body?
Ans: Calcium (Ca), vital for bones and teeth.
Q3: Why is beryllium different from other group members?
Ans: Due to small size, high ionization energy, and covalent bonding tendency.
