IntroductionHaloalkanes and Haloarenes
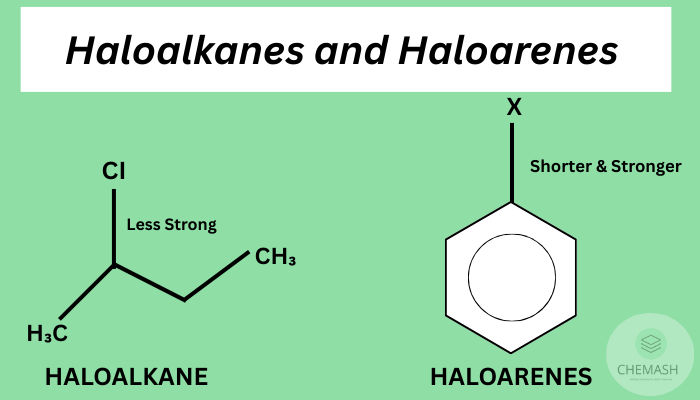
Haloalkanes and haloarenes are organic compounds containing halogen atoms (F, Cl, Br, I) bonded to aliphatic and aromatic hydrocarbons, respectively. They are important intermediates in organic synthesis, pharmaceuticals, and industrial chemistry.
Classification
1. Based on number of halogen atoms
- Monohalo: One halogen (e.g., CH₃Cl)
- Dihalo: Two halogens (e.g., CH₂Cl₂)
- Polyhalo: More than two (e.g., CCl₄)
2. Based on hydrocarbon type
- Haloalkanes (alkyl halides): Halogen bonded to saturated carbon (e.g., CH₃CH₂Cl).
- Haloarenes (aryl halides): Halogen bonded to aromatic ring (e.g., C₆H₅Cl — chlorobenzene).
Nomenclature
Follow IUPAC: identify the longest chain, number to give lowest locant to the halogen, and use prefixes fluoro-, chloro-, bromo-, iodo-. Example: CH₃CH₂CH(Br)CH₃ → 2-bromobutane.
Useful reference: IUPAC • NCERT basics: ncert.nic.in
Methods of Preparation
- From alcohols: ROH + HX → RX + H₂O (or use SOCl₂ / PBr₃ / PCl₅).
- Halogenation of alkanes: RH + X₂ → R–X + HX (free-radical, UV initiated).
- Sandmeyer: Ar–N₂⁺X⁻ + CuX → Ar–X + N₂ (for aryl halides).
- From alkenes: Addition of HX or halogen (e.g., CH₂=CH₂ + HBr → CH₃CH₂Br).
Physical Properties
- Boiling points increase with molecular mass and halogen size (I > Br > Cl > F).
- More polar than alkanes (C–X dipole) but less than alcohols.
- Generally insoluble in water; soluble in organic solvents.
Chemical Reactions
1. Nucleophilic substitution (SN1 & SN2)
- SN2: Bimolecular, one-step; favored by primary substrates and strong nucleophiles; inversion of configuration (Walden inversion).
- SN1: Unimolecular, two-step; favored by tertiary substrates and polar protic solvents; involves carbocation with possible racemization.
- Example: CH₃CH₂Br + OH⁻ → CH₃CH₂OH + Br⁻
2. Elimination (E2 & E1)
Under basic conditions alkyl halides undergo β-elimination to give alkenes.
Example: CH₃CH₂Br + alc. KOH → CH₂=CH₂ + HBr
3. Reactions with metals
- Grignard reagent: R–X + Mg (dry ether) → R–MgX (powerful carbon nucleophile).
- Wurtz coupling: 2 R–X + 2 Na → R–R + 2 NaX (coupling reaction).
Reactivity of Haloarenes
- Haloarenes are less reactive toward nucleophilic substitution because the halogen lone pair delocalizes into the aromatic π system, giving C–X partial double-bond character.
- They readily undergo electrophilic substitution (nitration, sulfonation, halogenation) — halogens are deactivating overall (−I) but ortho/para directing via +M.
- SNAr (nucleophilic aromatic substitution) can occur with strong −M groups (e.g., NO₂) at ortho/para positions via a Meisenheimer intermediate under harsh conditions.
Environmental Aspects
- Polyhalogenated compounds (DDT, PCBs, CFCs) are persistent, bioaccumulative and harmful to ecosystems.
- CFCs deplete stratospheric ozone — regulated internationally (Montreal Protocol).
- Proper disposal and green alternatives are important; see UNEP guidance.
Practice: MCQ, Quiz, Matching, True/False & Fill-ups
Multiple Choice Questions (MCQ)
- Which reagent converts alcohol to alkyl chloride efficiently?
- NaOH
- PCl5 / SOCl2
- H2, Pd
- KMnO4
- SN2 reactions are fastest with:
- Tertiary haloalkanes
- Secondary haloalkanes
- Primary haloalkanes
- Aryl halides
- Which statement about haloarenes is true?
- They undergo SN2 easily at room temp
- C–X bond has partial double bond character
- They are more reactive than haloalkanes in nucleophilic substitution
- They are always soluble in water
Quick Quiz (short answers)
- State one reason why SN2 is difficult on aryl halides. AnswerResonance gives C–X partial double bond character; ipso carbon is sp².
- Which elimination gives alkenes in one step? AnswerE2 (bimolecular elimination).
- Name the intermediate formed in SNAr. AnswerMeisenheimer (σ-complex).
Matching
- SN1
- SN2
- Grignard
- Sandmeyer
- Meisenheimer complex
- Forms RMgX
- Bimolecular inversion
- Unimolecular, carbocation
- Replacement of diazonium by halogen
- Intermediate in SNAr
Answer key
1→C, 2→B, 3→A, 4→D, 5→E
True / False
- Haloalkanes are generally soluble in water. — False. (They are insoluble; soluble in organic solvents.)
- SN1 involves a carbocation intermediate. — True.
- Halogens on benzene ring activate it for electrophilic substitution. — Partly true: They are deactivating overall (−I) but direct to ortho/para via +M.
Fill in the blanks
- In SN2 reactions, the stereochemistry at the reaction centre is _______. (Answer: inverted)
- Grignard reagents are prepared by reacting an alkyl halide with _______. (Answer: magnesium in dry ether)
- The reaction Ar–N₂⁺X⁻ → Ar–X + N₂ is called _______. (Answer: Sandmeyer reaction)
Frequently Asked Questions
Q: What is the key difference between haloalkanes and haloarenes?
A: Haloalkanes have halogen on saturated (sp³) carbons; haloarenes have halogen on aromatic (sp²) carbons — this dramatically alters reactivity (esp. toward nucleophiles). Q: Why do tertiary haloalkanes prefer SN1?
A: Tertiary carbocations are stabilized by alkyl groups (hyperconjugation + inductive), making the unimolecular ionization step feasible. Q: Are all halogenated compounds environmentally harmful?
A: Not all, but many polyhalogenated compounds (e.g., DDT, PCBs, CFCs) are persistent and hazardous. Regulation and proper disposal are essential. Q: Where can I read more?
हिंदी: संक्षिप्त नोट्स
हैलोअल्केन (Haloalkanes): अल्केन के हाइड्रोजन की जगह हैलोजन (F, Cl, Br, I) होने पर बनते हैं — सामान्य सूत्र R–X।
मुख्य बिंदु:
- SN2 प्राथमिक श्रेणी पर तेज़; SN1 तृतीयक पर तेज़।
- Grignard: R–X + Mg → R–MgX (सूखा एथर में)।
- हैलोएरीन्स (Aryl halides): रेज़ोनेंस के कारण न्यूक्लियोफिलिक सब्स्टिट्यूशन मुश्किल।
Takeaway: Haloalkanes are reactive in substitution & elimination reactions depending on structure (1°, 2°, 3°). Haloarenes resist nucleophiles due to resonance but undergo electrophilic substitution.
Related: Haloarenes — Detailed Notes •
External: Wikipedia: Haloalkane
