Ideal Gas Law
The Ideal Gas Law (PV = nRT) is a fundamental relationship in chemistry that connects pressure, volume, temperature, and moles of a gas. It combines Boyle’s, Charles’s, and Avogadro’s laws into one equation, making it a powerful tool for understanding gases under ideal conditions.
Table of Contents
- Equation & Variables
- Derivation of Ideal Gas Law
- Molecular Perspective
- Real-Life Applications
- Limitations
- Example Problem
- Quick Reference Table
- Quiz
- MCQs
- FAQs
Equation & Variables
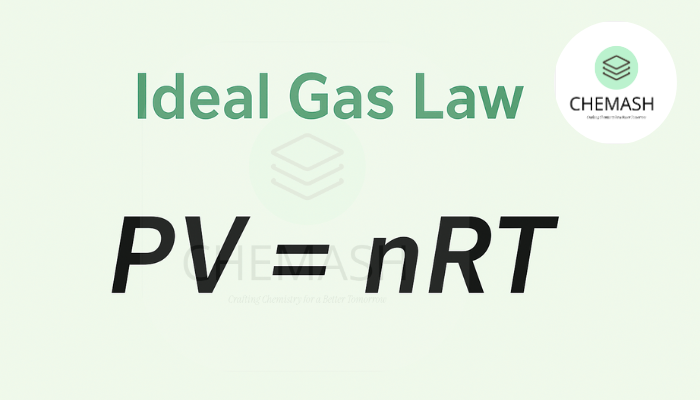
The Ideal Gas Law is expressed as:
PV = nRT
- P = Pressure (atm or Pa)
- V = Volume (L or m³)
- n = Number of moles
- R = Ideal Gas Constant (learn more)
- T = Temperature in Kelvin
Derivation of Ideal Gas Law
- Boyle’s Law: P ∝ 1/V (T constant)
- Charles’s Law: V ∝ T (P constant)
- Avogadro’s Law: V ∝ n (T,P constant)
Combining: V ∝ nT/P → PV = nRT
Molecular Perspective
- Gas molecules have negligible volume.
- No intermolecular forces.
- Collisions are perfectly elastic.
Real-Life Applications
- Airbags: Gas expansion after a chemical reaction.
- Ventilators: Modeling lung pressure and volume.
- Weather Balloons: Expansion with altitude.
- SCUBA Diving: Pressure & air consumption calculations.
Limitations
- Fails at high pressure & low temperature.
- Assumes point particles and no attractions.
- Corrections made using the Van der Waals Equation.
Example Problem
Q: Calculate moles in 10 L container at 2 atm, 300K (R = 0.0821 L·atm/mol·K).
Solution: n = (2×10) / (0.0821×300) ≈ 0.812 mol
Quick Reference Table
| Constant | Symbol | Value | Units |
|---|---|---|---|
| Ideal Gas Constant | R | 0.0821 | L·atm/mol·K |
| Avogadro’s Number | NA | 6.022 × 10²³ | particles/mol |
Quiz
- What does R represent?
Answer: Gas Constant
Explanation: Proportionality constant in PV = nRT. - If T increases at constant V?
Answer: Pressure increases
Explanation: PV = nRT, so P ∝ T. - When does Ideal Gas Law fail?
Answer: Low T, High P
Explanation: Intermolecular forces become significant.
MCQs
- Which laws combine to form Ideal Gas Law?
a) Boyle, Charles, Avogadro ✅ - Unit of R in SI?
a) 8.314 J/mol·K ✅ - Which gas behaves most ideally?
a) He ✅
FAQs
Q1: What is Ideal Gas Law used for?
A: To relate pressure, volume, temperature, and moles of gases.
Q2: When do real gases deviate?
A: At high pressure and low temperature.
Q3: Is any gas truly ideal?
A: No, but many approximate ideal behavior at low pressure & high temperature.

GOOD INFORMATIVE