Packing in Solids: Types, Efficiency, and Structures (SCP, BCC, FCC, HCP)
In the solid state, atoms, ions, or molecules are packed in a specific, highly ordered arrangement to occupy the minimum possible space. This arrangement affects the density, stability, and physical properties of the material. The study of how particles are arranged is known as crystal packing. Packing in Solids
Types of Packing in Solids
There are mainly two-dimensional (2D) and three-dimensional (3D) types of packing:
1. Two-Dimensional Packing
This occurs in a single layer of particles. There are two types:
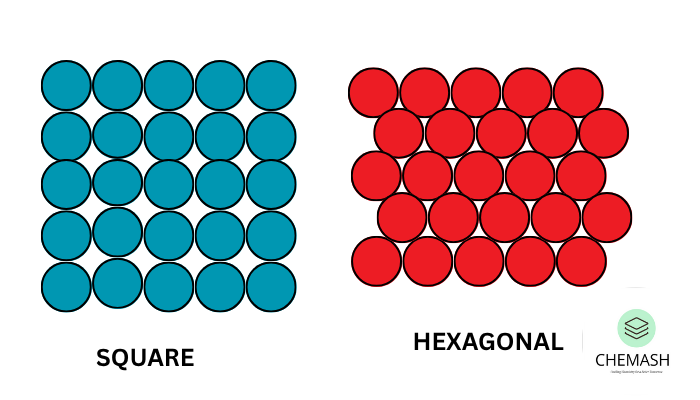
- Square Close Packing: Particles are arranged in rows and columns, directly aligned. This is less efficient.
- Hexagonal Close Packing (2D): Each particle is surrounded by six others. This arrangement is more efficient and denser.
2. Three-Dimensional Packing
Involves stacking multiple 2D layers to form a solid structure. There are mainly three types of 3D packing:
a. Simple Cubic Packing (SCP)
- Layers are stacked directly on top of each other: A-A-A type arrangement.
- Coordination number: 6
- Packing efficiency: ~52%
b. Body-Centered Cubic Packing (BCC)
- Particles are present at the corners and one in the center of the cube.
- Coordination number: 8
- Packing efficiency: ~68%
c. Close Packing (HCP & CCP/FCC)
Most efficient arrangements found in metals and crystals:
- Hexagonal Close Packing (HCP): Layer sequence: A-B-A-B
- Cubic Close Packing / Face-Centered Cubic (CCP/FCC): Layer sequence: A-B-C-A-B-C
- Coordination number: 12
- Packing efficiency: ~74%
Packing Efficiency
Packing efficiency refers to the percentage of total space occupied by particles in a crystal lattice.
| Packing Type | Coordination Number | Efficiency |
|---|---|---|
| Simple Cubic | 6 | 52% |
| Body-Centered Cubic (BCC) | 8 | 68% |
| FCC/CCP or HCP | 12 | 74% |
Conclusion: Efficient packing in solids helps minimize empty space and maximize stability. Metals and ionic solids typically exhibit close packing to achieve higher density and better physical properties.
Multiple Choice Questions (MCQs)
- Which of the following has the highest packing efficiency?
A. Simple Cubic
B. Body-Centered Cubic
C. Hexagonal Close Packing (HCP)
D. None of the above
Answer: C. Hexagonal Close Packing (HCP) - What is the coordination number in a face-centered cubic (FCC) structure?
A. 4
B. 6
C. 8
D. 12
Answer: D. 12 - Which arrangement is the least space-efficient?
A. BCC
B. FCC
C. Simple Cubic
D. HCP
Answer: C. Simple Cubic
True or False
- 1. In CCP arrangement, the particles are aligned in A-B-A-B layers.
Answer: False. CCP has an A-B-C-A-B-C arrangement. - 2. Packing efficiency of BCC is more than SCP but less than FCC.
Answer: True - 3. Coordination number in BCC is 6.
Answer: False. It is 8.
Explore More Solid State Topics
Density of Unit Cell // Imperfections in Solids Magnetic Properties
