Properties of Liquids
Liquids are an intermediate phase between solids and gases. Explore fluidity, viscosity, surface tension, capillary action, vapor pressure, MCQs, quiz with explanations, and an FAQ for quick revision. Properties of Liquids
Contents
- 1. Fluidity
- 2. Viscosity
- 3. Surface Tension
- 4. Capillary Action
- 5. Vapor Pressure & Boiling Point
- MCQs
- Quick Quiz (Printable)
- FAQ
- References & Links
1. Fluidity
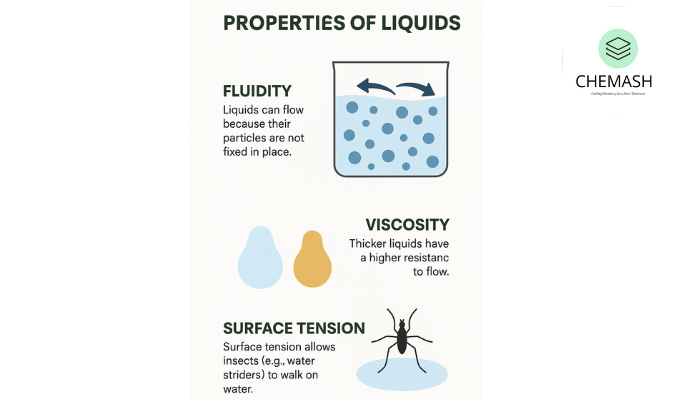
Liquids can flow because their particles are not fixed in place. Unlike solids, particles in a liquid can move past each other — allowing the liquid to adopt the shape of its container. Applications: hydraulics, lubricants, and circulation in biological systems.
2. Viscosity
Viscosity is the internal resistance of a liquid to flow. Honey is more viscous than water. Viscosity depends on molecular size, shape, and intermolecular forces.
| Factor | Effect |
|---|---|
| Molecular size/shape | Larger or more entangled molecules → higher viscosity |
| Intermolecular forces | Stronger forces → higher viscosity |
| Temperature | Higher temperature → lower viscosity |
Note: Increasing temperature increases kinetic energy and typically lowers viscosity.
3. Surface Tension
Surface tension is the energy required to increase the surface area of a liquid, caused by cohesive forces between molecules. It explains the spherical shape of droplets and why small insects (water striders) can walk on water.
Temperature effect: Surface tension decreases as temperature increases.
4. Capillary Action
Capillary action is the ability of a liquid to flow in narrow spaces without external forces. It arises from the balance of cohesive and adhesive forces and is central to processes such as water transport in plant xylem and ink movement in pens.
- Cohesion: Attraction between like molecules (liquid–liquid).
- Adhesion: Attraction between different materials (liquid–solid).
5. Vapor Pressure & Boiling Point
Vapor pressure is the pressure of a vapor in equilibrium with its liquid at a particular temperature. Liquids with higher vapor pressures evaporate more rapidly (for example, ethers and alcohols).
Boiling point: The temperature at which a liquid’s vapor pressure equals the surrounding atmospheric pressure. At high altitude (lower atmospheric pressure), liquids boil at lower temperatures.
MCQs — Check your understanding
- Which property allows insects to walk on water?
a) Viscosity
b) Surface tension
c) Capillary action
d) Fluidity
Answer: b) Surface tension — creates a strong surface layer on water, allowing insects to walk. - What happens to viscosity of a liquid as temperature increases?
a) Increases
b) Decreases
c) Remains the same
d) Doubles
Answer: b) Decreases — Temperature increases molecular motion, reducing intermolecular attractions. - Capillary rise occurs due to:
a) Cohesion
b) Adhesion
c) Both a and b
d) Surface tension alone
Answer: c) Both a and b — Capillary action results when adhesive and cohesive forces act together. - Which liquid property determines its evaporation rate?
a) Viscosity
b) Vapor pressure
c) Surface tension
d) Density
Answer: b) Vapor pressure — Higher vapor pressure means faster evaporation.
Quick Quiz (Printable)
- Define surface tension and give one real-world example.
- Explain why honey flows slower than water.
- How does atmospheric pressure affect the boiling point of water?
- Describe capillary action and its role in plants.
Answers & Explanations
- Surface tension is the energy required to increase surface area of a liquid. Example: droplets forming spheres.
- Honey has stronger intermolecular interactions and larger molecules, so viscosity is higher.
- Lower atmospheric pressure reduces the boiling point since vapor pressure equals ambient pressure sooner.
- Capillary action is due to adhesion + cohesion; in plants it helps water rise through xylem vessels.
FAQ — Quick answers
Q: Does surface tension affect detergent performance?
A: Yes — detergents reduce surface tension, allowing water to wet surfaces and remove oil and dirt more effectively. Read more.
Q: Why do some liquids evaporate faster than others?
A: Evaporation rate depends mainly on vapor pressure at the given temperature; higher vapor pressure means faster evaporation. More detail.
Q: Is viscosity measured in SI units?
A: Yes — dynamic viscosity is measured in pascal-seconds (Pa·s) in SI; centipoise (cP) is common in labs. Learn more.
Published by CHEMASH — last updated: Oct 2, 2025

Pingback: Properties of Solids - CHEMASH
informative