Redox Titrations
Chemists use redox titrations to determine the concentration of unknown substances through oxidation–reduction reactions. These titrations depend on the transfer of electrons between the titrant and analyte. Unlike acid–base titrations, they rely on electron balance rather than proton transfer.
Principle of Redox Titrations
In a redox titration, an oxidizing agent reacts with a reducing agent. The equivalence point occurs when electrons lost equal electrons gained. Therefore, stoichiometry guides the calculation of the unknown concentration.
Types of Redox Titrations

- Potassium permanganate titrations (KMnO₄) — act as self-indicators and provide strong oxidation power.
- Dichromate titrations (K₂Cr₂O₇) — require external indicators such as diphenylamine.
- Thiosulfate titrations (Na₂S₂O₃) — play a major role in iodometry.
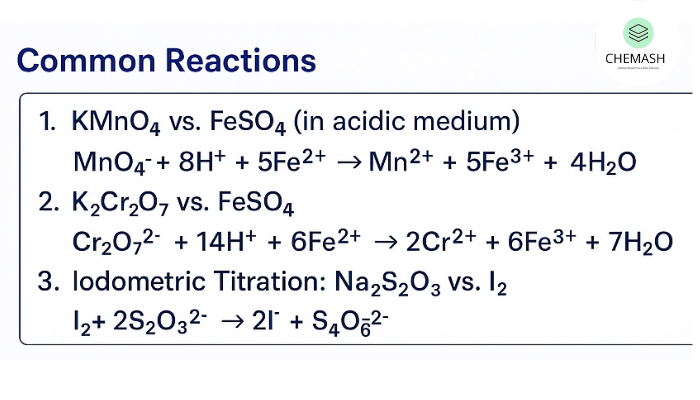
Common Reactions (Balanced Equations)
1. KMnO₄ vs FeSO₄ (acidic medium)
MnO₄⁻ + 8H⁺ + 5Fe²⁺ → Mn²⁺ + 5Fe³⁺ + 4H₂O
2. K₂Cr₂O₇ vs FeSO₄ (acidic medium)
Cr₂O₇²⁻ + 14H⁺ + 6Fe²⁺ → 2Cr³⁺ + 6Fe³⁺ + 7H₂O
3. Iodometric titration (thiosulfate vs iodine)
I₂ + 2S₂O₃²⁻ → 2I⁻ + S₄O₆²⁻
Indicators and End-Point Detection
- KMnO₄: serves as its own indicator. The solution changes from purple to colorless, and the endpoint appears as a faint permanent pink.
- Starch: forms a deep blue complex with iodine. The disappearance of this color marks the endpoint in iodometry.
- Diphenylamine: works as an external indicator in dichromate titrations.
Quick Quiz
- What color change do you observe when KMnO₄ reduces? AnswerPurple to colorless.
- Which reagent usually works with starch indicator? AnswerIodine (I₂).
- What role does Fe²⁺ play in KMnO₄ titration? AnswerFe²⁺ acts as the reducing agent and oxidizes to Fe³⁺.
- Name one titration that needs no external indicator. AnswerKMnO₄ titration, because it is a self-indicator.
- What marks the endpoint in iodometric titration? AnswerThe disappearance of the blue starch–iodine complex.
MCQs
- Which of the following is a self-indicator?
a) K₂Cr₂O₇
b) KMnO₄
c) HCl
d) Na₂S₂O₃ Correct: b) KMnO₄ - Which indicator detects the endpoint in iodometric titrations?
a) Phenolphthalein
b) Methyl orange
c) Starch
d) Litmus Correct: c) Starch - What is the oxidation state of Mn in KMnO₄?
a) +2
b) +4
c) +7
d) +6 Correct: c) +7 - In redox titrations, when does the equivalence point occur?
a) When oxidant volume is minimized
b) When electrons gained equal electrons lost
c) When pH = 7
d) When concentrations are equal Correct: b) Electrons gained = electrons lost - Fe²⁺ reacts with KMnO₄ in which medium?
a) Basic
b) Acidic
c) Neutral
d) Ammoniacal Correct: b) Acidic
FAQs
Why do we use acidic medium with KMnO₄?
Acidic medium (H₂SO₄) prevents MnO₂ precipitation and allows MnO₄⁻ to reduce cleanly to Mn²⁺.
How can you calculate the amount of substance in titration?
You apply stoichiometry from the balanced equation. Then, multiply the titrant’s concentration by its volume to compute moles of analyte.
Can chemists apply iodometry to oxidizing agents?
Yes. Many oxidizing agents release iodine from iodide, and you can titrate the iodine with thiosulfate.
Published: Sep 29, 2025 • Category: Analytical Chemistry
