Aromatic Hydrocarbons — Comprehensive Overview
Table of Contents:
- Introduction
- What is Aromaticity?
- Structure of Benzene
- Properties
- Types of Aromatic Hydrocarbons
- Reactions
- Applications
- Environmental & Health Aspects
- Quiz Section
- FAQs
Introduction to Aromatic Hydrocarbons
Aromatic hydrocarbons, or arenes, are a special class of organic compounds distinguished by the presence of one or more planar cyclic rings with conjugated π-electron systems that exhibit aromaticity. This unique property imparts remarkable stability to these compounds.
The term “aromatic” originated from the fragrant nature of many such compounds, though today it refers to their electronic structure and stability, not odor.
What is Aromaticity?
Aromaticity describes the exceptional stability of cyclic, planar molecules with continuous overlapping p-orbitals containing 4n + 2 π electrons (Hückel’s Rule).
- Cyclic and planar molecule
- Continuous overlap of p-orbitals
- 4n + 2 π electrons (n = 0,1,2,…)
Structure of Benzene — Prototype Aromatic Compound
Benzene (C₆H₆) is a planar hexagonal ring with delocalized π electrons evenly spread across six carbons. All C–C bonds are equal (~1.39 Å), depicted as a circle inside the hexagon.
Properties of Aromatic Hydrocarbons
- Colorless liquids with distinctive odors
- Insoluble in water, soluble in organic solvents
- Resistant to addition reactions
- Boiling points rise with molecular weight
- Some exhibit electrical conductivity
Common Types of Aromatic Hydrocarbons
- Monocyclic: Benzene, Toluene, Phenol
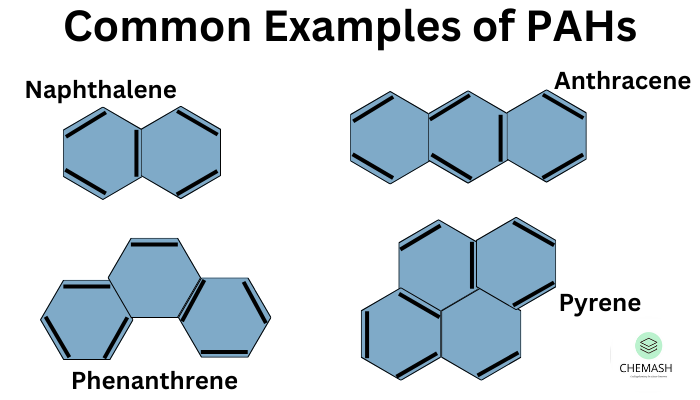
- Polycyclic Aromatic Hydrocarbons (PAHs): Naphthalene, Anthracene, Phenanthrene
- Heteroaromatic (not hydrocarbons): Pyridine, Furan
Reactions of Aromatic Hydrocarbons
Electrophilic Aromatic Substitution (EAS)
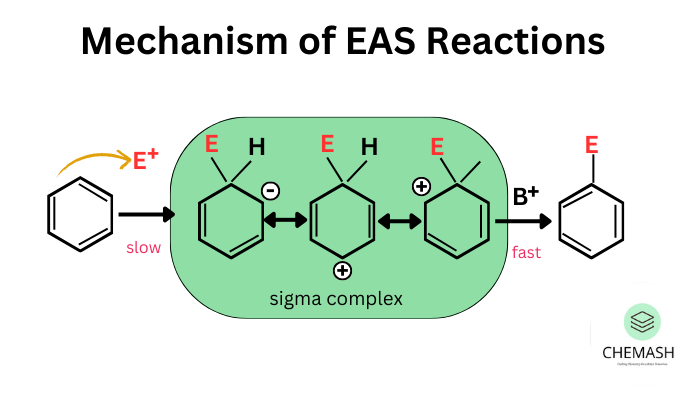
- Nitration — introduces –NO₂
- Halogenation — Cl/Br substitution
- Sulfonation — –SO₃H introduction
- Friedel-Crafts Alkylation & Acylation
Addition Reactions
Occur under special conditions (e.g., hydrogenation).
Side Chain Reactions
Oxidation of toluene → benzoic acid.
Applications and Importance
- Precursors for dyes, drugs, plastics, perfumes
- Used as solvents & fuels
- Building blocks in polymers and nanomaterials
Environmental and Health Aspects
Some PAHs are toxic, carcinogenic pollutants from incomplete combustion. Safe handling and regulations are crucial.
Practice Quiz — Aromatic Hydrocarbons
MCQ
Which of the following follows Hückel’s Rule?
- A. Cyclobutadiene
- B. Benzene ✔
- C. Cyclooctatetraene
- D. None
Fill in the Blanks
Benzene has ____ delocalized π electrons. (Answer: 6)
True/False
Benzene readily undergoes addition reactions. False
Frequently Asked Questions
Q1: Why is benzene more stable than expected?
Ans: Because of delocalized π electrons providing resonance stabilization.
Q2: Are all aromatic compounds fragrant?
Ans: No, “aromatic” refers to structure, not smell.
Q3: What are PAHs?
Ans: Polycyclic Aromatic Hydrocarbons with fused benzene rings, some being toxic.
Summary:
Aromatic hydrocarbons are stable, cyclic π-electron compounds obeying Hückel’s Rule. They are vital in industry but also pose environmental concerns.
Related topics: IUPAC Nomenclature, Reaction Mechanisms
External resource: LibreTexts Organic Chemistry
