Isotopes and Isobars
In atomic theory, the concepts of isotopes and isobars are essential to understanding variations in atomic structures and their behavior. These terms explain why atoms of the same or different elements can share some properties while differing in others.
What are Isotopes?
Isotopes are atoms of the same element that have the same number of protons (atomic number) but different numbers of neutrons. Thus, isotopes differ in their mass numbers but exhibit similar chemical properties due to identical electron configurations.
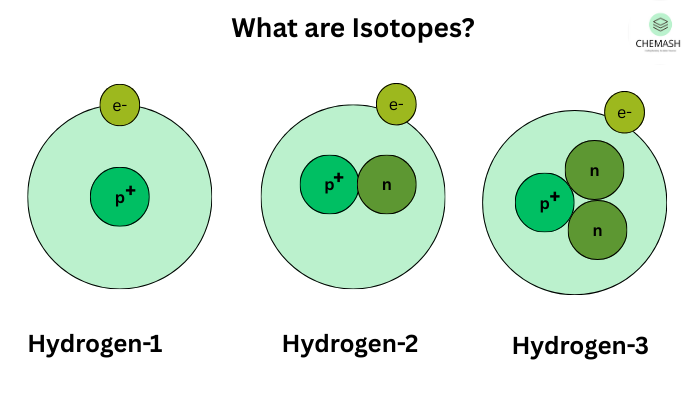
Examples of Isotopes:
- Hydrogen Isotopes:
- Protium (¹H): 1 proton, 0 neutrons
- Deuterium (²H or D): 1 proton, 1 neutron
- Tritium (³H or T): 1 proton, 2 neutrons
- Carbon Isotopes: ¹²C and ¹⁴C
- Uranium Isotopes: ²³⁵U and ²³⁸U
Figure: Isotopes of Hydrogen
What are Isobars?

Isobars are atoms of different elements that have the same mass number but different atomic numbers. They therefore have different numbers of protons and neutrons, leading to different chemical properties despite the same total mass.
Examples of Isobars:
- Argon-40 (⁴⁰Ar): 18 protons, 22 neutrons
- Calcium-40 (⁴⁰Ca): 20 protons, 20 neutrons
- Potassium-40 (⁴⁰K): 19 protons, 21 neutrons
Comparison Table
| Feature | Isotopes | Isobars |
|---|---|---|
| Atomic Number | Same | Different |
| Mass Number | Different | Same |
| Chemical Properties | Similar | Different |
MCQ
- What is the key difference between isotopes and isobars?
- a) Isotopes have the same mass, isobars have different atomic number
- b) Isotopes: same atomic number, different mass ✅
- c) Isobars: same chemical properties
- d) None of the above
- Which of the following are isotopes?
- a) ¹²C and ¹⁴C ✅
- b) ⁴⁰K and ⁴⁰Ca
- c) ¹²C and ⁴⁰Ca
- d) ⁴⁰K and ⁴⁰Ar
- Do isotopes of an element have the same chemical properties?
- a) Yes ✅
- b) No
- Which particle varies in isotopes?
- a) Proton
- b) Neutron ✅
- c) Electron
FAQs on Isotopes and Isobars
1. What are isotopes?
Atoms of the same element with the same atomic number but different mass numbers due to varying neutrons.
2. What are isobars?
Atoms of different elements with the same mass number but different atomic numbers.
3. Do isotopes have different chemical properties?
No, they exhibit almost identical chemical properties due to the same electronic structure.
4. Do isobars have similar properties?
No, since they belong to different elements, their chemical properties differ.
5. Where are isotopes used?
In medical imaging, radiotherapy, carbon dating, and tracer studies.
Related reading: Atomic Structure | Electronic Configuration | Encyclopedia Britannica on Isotopes
