Mass Spectrometry (MS) – Principle, Ionization, Mass Analyzer & Applications |
Mass Spectrometry (MS) is a highly sensitive analytical technique that chemists use to determine the molecular mass, elemental composition, and structural features of chemical compounds. Unlike spectroscopic methods based on electromagnetic radiation, mass spectrometry measures the mass-to-charge ratio of ions.
Moreover, within the CHEMASH spectroscopy SILO, mass spectrometry integrates closely with NMR Spectroscopy, Infrared Spectroscopy, and UV–Visible Spectroscopy. As a result, learners gain a complete and reliable approach to molecular identification.
Table of Contents
- Introduction to Mass Spectrometry
- Principle of Mass Spectrometry
- Ionization Techniques
- Mass Analyzers
- Detector System
- Mass Spectrum Interpretation
- Important Peaks in Mass Spectrum
- Fragmentation Pattern
- Applications of MS
- Advantages of Mass Spectrometry
- Limitations of MS
- Frequently Asked Questions
Introduction
Mass spectrometry plays a crucial role in analytical chemistry, organic chemistry, biochemistry, environmental science, and pharmaceutical research. Scientists actively use this technique to identify unknown compounds, confirm molecular formulas, and study isotopic distributions.
Furthermore, modern mass spectrometers offer extremely high sensitivity, often detecting compounds at nanogram or picogram levels. Therefore, MS has become indispensable in forensic analysis and trace-level investigations.
Principle
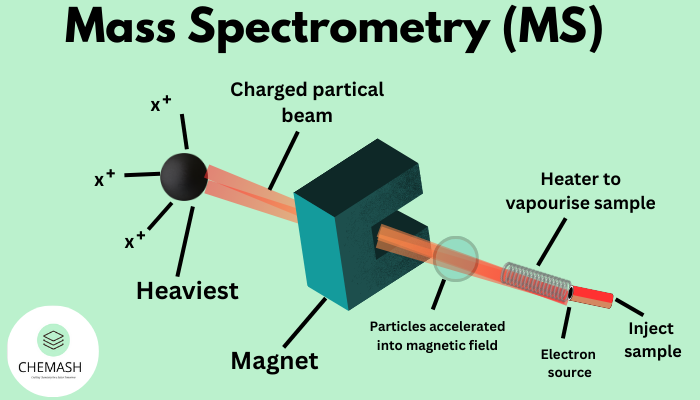
The principle of mass spectrometry involves converting molecules into charged particles, separating these ions according to their mass-to-charge ratio (m/z), and detecting them to generate a mass spectrum.
First, the sample undergoes ionization. Next, an electric or magnetic field accelerates and separates the ions. Finally, a detector records the abundance of each ion.
m/z = mass of ion / charge on ion
Because most ions carry a +1 charge, the m/z value often corresponds directly to the molecular mass.
Ionization Techniques
Ionization is a critical step because neutral molecules cannot be analyzed directly. Different ionization methods suit different types of compounds.
Electron Impact Ionization (EI)
Electron impact ionization uses high-energy electrons to bombard gaseous molecules. As a result, molecules lose electrons and form positive ions. EI produces extensive fragmentation and provides rich structural information.
Chemical Ionization (CI)
Chemical ionization is a softer technique compared to EI. It produces fewer fragments and enhances the molecular ion peak. Therefore, CI helps determine molecular mass more accurately.
Electrospray Ionization (ESI)
Electrospray ionization works well for polar and biomolecular compounds. It generates multiply charged ions and allows analysis of large molecules.
Matrix-Assisted Laser Desorption Ionization (MALDI)
MALDI uses laser energy and a matrix compound to ionize large biomolecules gently. Consequently, it is widely used in proteomics and polymer analysis.
Mass Analyzers in MS Instruments
Mass analyzers separate ions based on their m/z values. Each analyzer offers unique advantages in terms of resolution, speed, and accuracy.
- Magnetic Sector Analyzer – high accuracy
- Time-of-Flight (TOF) – rapid analysis
- Quadrupole Analyzer – compact and cost-effective
- Ion Trap – tandem MS capability
Detector System
After separation, ions strike a detector that converts ion impact into an electrical signal. The signal intensity reflects the abundance of ions.
Common detectors include electron multipliers and Faraday cups. Because of their high sensitivity, electron multipliers dominate modern instruments.
Mass Spectrum Interpretation
A mass spectrum plots ion intensity versus m/z value. Each peak corresponds to a specific ion formed during ionization or fragmentation.
By analyzing peak positions and relative intensities, chemists deduce molecular weight and structural features.
Important Peaks in a Mass Spectrum
- Molecular ion peak (M⁺) – represents intact molecule
- Base peak – most intense peak
- Fragment peaks – indicate breakdown pathways
- Isotopic peaks – reveal elemental composition
Fragmentation Pattern
Fragmentation occurs when molecular ions break into smaller ions. These patterns provide valuable structural clues.
For example, alkyl chains often show characteristic cleavage, while aromatic compounds display stable fragment ions. Therefore, fragmentation analysis strengthens structure confirmation.
Applications of Mass Spectrometry
Mass spectrometry supports diverse scientific and industrial applications. Additionally, it complements NMR and IR techniques for complete structure analysis.
- Determination of molecular mass
- Structural elucidation of organic compounds
- Pharmaceutical quality control
- Proteomics and metabolomics
- Environmental pollutant analysis
- Forensic investigations
Authoritative references: ScienceDirect, Britannica
Advantages
- Extremely high sensitivity
- Accurate molecular mass determination
- Small sample requirement
- Wide applicability
Limitations of Mass Spectrometry
Despite its power, mass spectrometry has limitations. Some compounds fragment excessively, making molecular ion detection difficult. Moreover, instrument cost remains high.
Frequently Asked Questions
What does mass spectrometry measure?
Mass spectrometry measures the mass-to-charge ratio of ions to determine molecular mass and structure.
Why is molecular ion peak important?
It provides direct information about the molecular weight of the compound.
Which ionization method is soft?
Chemical ionization and electrospray ionization are considered soft techniques.
© CHEMASH – Complete Spectroscopy Learning Hub
