Rate of Reaction (Chemical Kinetics)
The rate of reaction describes how quickly a chemical change takes place. In chemistry, this concept is studied under the branch called chemical kinetics. Understanding reaction speed is essential for laboratory experiments, industrial processes, biochemical reactions, and environmental systems.
Table of Contents
- Meaning of Reaction Rate
- Basic Concept of Chemical Kinetics
- Formula, Symbols & Units
- Classification of Reaction Rates
- Factors Affecting Speed of Reaction
- Reaction Rate Graphs
- Solved Numerical Problems
- Importance & Applications
- MCQs with Explanations
- Frequently Asked Questions
1. Meaning of Reaction Rate
The reaction rate is defined as the change in concentration of a reactant or a product per unit time during a chemical reaction. It gives a quantitative idea about how fast or slow a reaction proceeds.
Simple definition:
It measures the speed at which reactants are transformed into products.
Fast reactions such as ionic reactions occur almost instantly, while slow reactions like rusting of iron may take years to complete.
2. Concept of Chemical Kinetics
Chemical kinetics deals with the study of reaction rates, reaction mechanisms, and factors influencing the speed of chemical reactions. Unlike thermodynamics, kinetics does not tell whether a reaction is feasible, but explains how quickly it occurs.
For example, diamond is thermodynamically unstable compared to graphite, yet it converts extremely slowly due to a very low reaction rate.
3. Formula, Symbols and Units
General expression:
Reaction Rate = Change in concentration / Time interval
Mathematical form:
Rate = −Δ[Reactant] / Δt
Rate = +Δ[Product] / Δt
Units of Chemical Reaction Rate
| Parameter | Unit |
|---|---|
| Concentration | mol L⁻¹ |
| Time | seconds (s) |
| Reaction rate | mol L⁻¹ s⁻¹ |
4. Classification of Reaction Rates
- Average rate – measured over a finite time interval
- Instantaneous rate – measured at a particular moment
- Initial rate – rate at the beginning of the reaction
Instantaneous reaction speed is obtained from the slope of the tangent on a concentration–time graph.
5. Factors Affecting Speed of Chemical Reactions
| Factor | Explanation |
|---|---|
| Concentration | Higher concentration increases collision frequency |
| Temperature | Increases kinetic energy of molecules |
| Catalyst | Provides an alternative low-energy pathway |
| Surface area | More exposed particles react faster |
| Nature of reactants | Ionic reactions are usually faster |
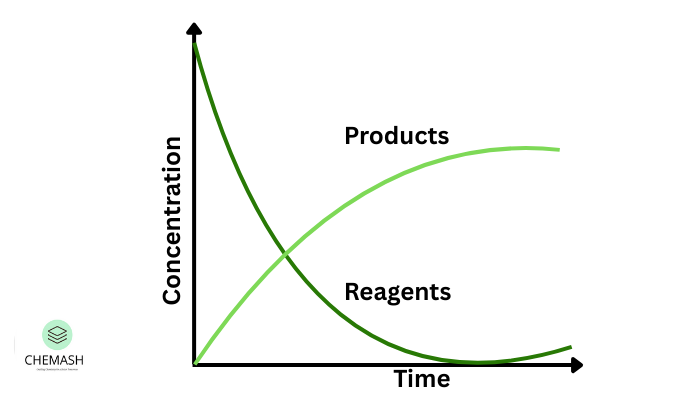
6. Reaction Rate Graphs
Graphs between concentration and time are commonly used to analyze reaction kinetics.
- Steep slope → high reaction speed
- Gentle slope → slow reaction
- Slope at a point → instantaneous rate
The decreasing slope over time indicates reduction in reactant concentration.
7. Solved Numerical Example
Problem: The concentration of a reactant drops from 1.0 mol L⁻¹ to 0.4 mol L⁻¹ in 40 seconds. Calculate the reaction speed.
Solution:
ΔC = 1.0 − 0.4 = 0.6 mol L⁻¹
Δt = 40 s
Reaction rate = 0.6 / 40 = 0.015 mol L⁻¹ s⁻¹
8. Importance and Applications
- Design of industrial reactors
- Manufacture of pharmaceuticals
- Food processing and preservation
- Environmental pollution control
- Understanding biological enzyme reactions
9. Multiple Choice Questions
Q1. The unit of reaction speed is:
- A. mol L⁻¹
- B. mol s⁻¹
- C. mol L⁻¹ s⁻¹ ✅
- D. L mol⁻¹ s⁻¹
Explanation: Rate is concentration change per unit time.
Q2. Which factor does not influence chemical kinetics?
- A. Temperature
- B. Catalyst
- C. Concentration
- D. Colour of substance ✅
10. Frequently Asked Questions
What is meant by reaction rate?
It indicates how fast reactants are converted into products.
Why does temperature increase reaction speed?
Higher temperature increases molecular collisions and energy.
Does reaction speed remain constant?
No, it usually decreases as reactants are consumed.
Khan Academy – Chemical Kinetics
