Redox Reactions in Acidic and Basic Medium
Redox reactions often take place in either an acidic or a basic aqueous medium. The method for balancing these reactions depends on the medium and involves H+, OH– and H2O to account for hydrogen and oxygen atoms.
Redox Reactions in Acidic Medium
In acidic medium use H+ and H2O when balancing. Steps (half-reaction method):
- Separate into oxidation and reduction half-reactions.
- Balance atoms other than H and O.
- Balance O using H2O.
- Balance H using H+.
- Balance charge with electrons (e–).
- Multiply to equalize electrons and combine; cancel common species.
Example (Acidic Medium)
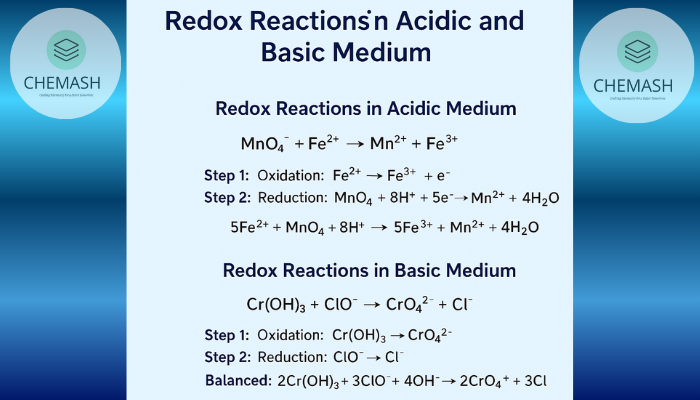
MnO₄⁻ + Fe²⁺ → Mn²⁺ + Fe³⁺ Step 1: Oxidation: Fe²⁺ → Fe³⁺ + e⁻ Step 2: Reduction: MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O Final: 5Fe²⁺ + MnO₄⁻ + 8H⁺ → 5Fe³⁺ + Mn²⁺ + 4H₂O
Redox Reactions in Basic Medium
In basic medium use OH– and H2O. Follow acidic steps, then remove H+ by adding OH– to both sides and convert to water.
Example (Basic Medium)
Cr(OH)₃ + ClO⁻ → CrO₄²⁻ + Cl⁻ Step 1: Oxidation: Cr(OH)₃ → CrO₄²⁻ Step 2: Reduction: ClO⁻ → Cl⁻ Balanced: 2Cr(OH)₃ + 3ClO⁻ + 4OH⁻ → 2CrO₄²⁻ + 3Cl⁻ + 5H₂O
Key Differences
| Aspect | Acidic Medium | Basic Medium |
|---|---|---|
| Ions used | H+ | OH– |
| Neutralization | With H2O only | H+ + OH– → H2O |
| pH range | < 7 | > 7 |
Quiz: Redox in Acidic & Basic Medium
- What ion is used to balance H atoms in an acidic medium? AnswerH+
- What is the purpose of OH– in redox balancing? AnswerTo neutralize H+ and convert acidic-balanced equations to basic form.
- Which ion reacts with H+ to form H2O? AnswerOH–
- Is ClO– an oxidizing or reducing agent in basic medium? AnswerOxidizing agent.
- What is the product of MnO₄– reduction in acidic solution? AnswerMn²⁺
MCQs
- In basic medium, H+ ions are neutralized by:
a) Cl⁻ b) SO₄²⁻ c) OH⁻ d) H₂Correct: c) OH⁻ - Which ion is added to balance O in acidic medium?
a) OH⁻ b) H₂O c) H+ d) O₂Correct: b) H₂O - Which is correct in basic redox balancing?
a) Add H+ to both sides b) Subtract OH⁻ from products c) Add OH⁻ to cancel H+ d) Add O₂ gasCorrect: c) Add OH⁻ to cancel H+ - In MnO₄⁻ → Mn²⁺ in acidic medium, Mn is:
a) Oxidized b) Reduced c) No change d) DisproportionatedCorrect: b) Reduced - In acidic medium, Cl⁻ → Cl₂ is:
a) Reduction b) Oxidation c) Substitution d) HydrolysisCorrect: b) Oxidation
- Oxidation and Reduction — CHEMASH
- Oxidation Number — CHEMASH
- LibreTexts Chemistry
- Britannica: Oxidation–reduction reaction
FAQs
How do I balance redox reactions in acidic medium?
Use the half-reaction method: balance non-H/O atoms, then O with H2O, H with H+, balance charge with electrons, combine and simplify.
How do I change an acidic-balanced equation to basic?
Add OH- equal to the number of H+ to both sides, form water, then cancel waters.
What is disproportionation?
When a single species undergoes both oxidation and reduction simultaneously (e.g., 2H2O2 → 2H2O + O2).
Published: Sep 29, 2025 • Category: Redox Reactions
